research notes, discussions, events, contributions related to applied colloid sciences - characterization of dispersions, personal views to general topics news related to http://www.AppliedColloidsSurfactants.info Profile at LinkedIn http://www.linkedin.com/pub/titus-sobisch/32/524/293
Popular Posts
-
Water and Wastewater.com Help Forum - Tertiary Treatment Surfactant Foam Question by Hilary - CAMS We have a modified UCT BNR plant ...
-
--> Characterization of the dispersion properties of carbon nanotubes in ionic liquids by the separation behaviour in the centrifuga...
-
Scope Carbon blacks are widely applied as pigments and fillers in various products (inks, paints, rubber, plastics). A multisampl...
-
Scope Selection of emulsifiers and evaluation of emulsion stability is a frequent task. This relates to practical issues like formu...
-
CME 2006 World Congress of Emulsions 3 - 6 Oct. 2006, Lyon, France Evaluation of long term stability of model emulsions by multisample analy...
Friday, April 15, 2005
Water and Wastewater.com Help Forum - Why need flocculation in DAF?
only to elaborate the influence besides the surface of flocs for capture.
Flotation can also be used to remove fine particles provided they have a hydrophobic surface. This is not the case for normal wastewater colloids and fines because they are usually negatively charged. By flocculation or coagulation these charges are equalized and the particles become less polar. Nevertheless the main removal mechanism is the formation of flocs.
Thursday, April 14, 2005
LUMiFuge 116 - Application - Evaluation of long-term stability of cosmetic creams
Scope
Multisample analytical centrifugation is efficient in characterization and measurement of the speed of destabilizing processes like creaming, coalescence and phase separation [1-3]. It detects very small differences in stability and allows for an accelerated characterization of emulsions without dilution, thus avoiding changes of emulsion properties.
Analytical centrifugation traces the inherent stability, but cannot foresee destabilizing processes that will happen during storage of the sample. But, it will see the results of destabilizing processes far more faster than visual observation. Therefore, to this end a combination with suitable stress tests is the method of choice.
As a demonstration the evaluation of aging of cosmetic creams is presented, which is important in relation to development of new formulations and in quality control of manufactured products. The stability of samples aged during storage at ambient conditions or by a freeze-thaw cycle (accelerated aging) was compared with the initial stability just after manufacturing.
Measurement principle
The Lumifuge 116 employs the STEP technology, which allows to measure the intensity of the transmitted light as function of time and position over the full sample length simultaneously. (Measurement scheme see Fig. 1)
The data are displayed as function of the radial position, as distance from the centre of the rotation (transmission profiles, see Fig. 2).
At the same time up to 8 different samples can be analysed simultaneously at temperatures up to 60 °C.
By means of the available analysis modes ‘Integral Transmission’ (Clarification) and ‘Front Tracking’ the separation behaviour of the individual samples can be compared and analysed in detail.
Experimental/Results
|
|
|
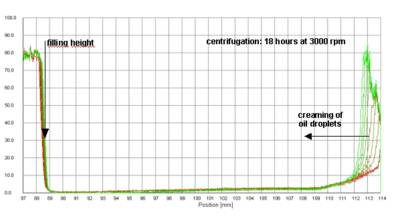
Fig. 2 Evolution of transmission profiles with time - first recorded profile undermost (red), last profile uppermost (green), centrifugation of a cosmetic cream of the o/w type at 1100 x g for 18 hours
The sharp drop in transmission at about 88.5 mm marks the filling height of the sample.
The lowest transmission belongs to the first profile (red). The destabilizing process, separation of an aqueous phase, starts from the bottom of the cell. The boundary water -emulsion is moving upwards (last profile green). That is, the separation process is characterized by creaming of oil droplets inside the continuous aqueous phase. From this it becomes obvious that the cream is an oil-in-water emulsion.
It has to be noted that the cream has a high inherent stability, given the long time and high speed of centrifugation necessary to produce these changes.
The more stable creams exhibited only a small increase of transmission near the cell bottom.
For a comparative investigation therefore the change in transmission of the bottom region was chosen as a measure of stability.
Figure 3 shows the effect of two month of aging at ambient temperature for the most and for the least stable sample.
The just prepared samples are clearly less stable than the samples after 2 month aging at ambient laboratory conditions.

Fig. 3 Change of integral transmission (113 - 114 mm) during centrifugation at 1100 x g for 18 hours - effect of 2 month aging at ambient temperature
However, further flocculation is expected to cause irreversible destabilization. In contrast other aging processes like coalescence and Ostwald Ripening would have led to a direct destabilization.
The effect of accelerated aging by a freeze-thaw cycle (thawing after 24 hours of storage at
-10 °C, compared to the samples not subjected to the freeze-thaw cycle) is depicted in

Fig. 4 Change of integral transmission (113 - 114 mm) during centrifugation at 1100 x g for 18 hours - effect of a freeze-thaw cycle after 2 month aging at ambient temperature
It is readily obvious from this comparison that aging by one freeze-thaw cycle markedly enhances the differences in stability already inherent in the samples before freezing.
As seen the separation of the continuous phase at the bottom is initially slow and speeds up only after a prolonged time of centrifugation. This indicates a stabilizing barrier against creaming (probably a flocculated network). The freeze-thaw cycle obviously reduces the stabilizing barrier causing a marked reduction of the induction period.
In the case investigated the order of stability of the different creams does not change during aging (2 month, or after an additional freeze-thaw cycle), i.e. the inherent stability of the samples as prepared is altered during aging for all samples the same way.
To trace slow destabilizing processes a combination with common accelerated aging tests is the method of choice
References
[1] Characterization and optimization of emulsions Chemistry Preprint Archive, Volume 2002, Issue 6, June 2002, Pages 195-201 [3] Rapid characterization of emulsions for emulsifier selection, quality control and evaluation of stability using multisample analytical centrifugation, T. Sobisch, D. Lerche, 2005, SCI/RSC/SCS conference Cosmetics and Colloids, London 15 February 2005 http://www.soci.org/SCI/groups/col/2005/reports/pdf/gs3257_sob.pdf[2] Stability analyser LUMiFuge 116 for rapid evaluation of emulsion stability and demulsifier selection,
D. Lerche, T. Sobisch, S. Küchler, 2002, http://www.sciencedirect.com/preprintarchive
Thursday, April 07, 2005
Biosurfactants for remediation of petroleum contamination - From the bioremediationgroup.org
Dr Naresh Singhal, Department of Civil and Environmental Engineering, University of Auckland
'I am looking for a company that sells bacterially derived biosurfactants. Ordinarily, I would have purchased these from Jeneil Biosurfactants in the US, ...... I need small quantities of these biosurfactants for use in our research.'
extended answer by
Valerie Anne Edwards, President, Alken-Murray Corporation
'The only problem with Pseudomonas derived biosurfactants is that they
kill Bacillus, while enhancing gram-negatives, especially Pseudomonas.
I ended up using Stepan coconut derived surfactants (Cocomidopropyl betaine
and coconut MEA and Desert King's Yucca schidigera formulas (DK
Sarsaponin 30, soluble Yucca schidigera 50 and Ag-Aide 50 liquid),
compatible with Bacillus, Pseudomonas, Marinobacter, Starkeya,
Paracoccus and Thiobacillus strains that I use in various bioremediation
projects and odor control applications. The performance of these
surfactants, their reasonable pricing and biodegradability are all
assets.
I also had considered Jeneil biosurfactants, but I only use liquid
surfactants in Bacillus based products and the antibacterial quality of
the Jeneil products ruined my interest in them.
...........
I have several Bacillus that tested positive
using the procedure of Adria A. Boudour and Raina M. Miller-Maier in
"Application of a Modified Drop-Collapse Technique for Surfactant
Quantification and Screening of Biosurfactants", following pre-screening
using blood agar, according to other procedures I have read, since this
is an easy and rapid test procedure. I do not separate enzymes or
biosurfactants from my Bacillus strains, but by adding Yucca schidigera
(accepted as a biological catalyst or nutrient in jurisdictions that
prohibit surfactant use) along with surfactant-producing Bacillus,
enables me to comply with the law in those localities, while obtaining
optimal results. My Alken Enz-Odor 2 is one such product.
You might want to test some surfactants derived from Yucca and coconut to
see if they will solve your requirements.
Another note is about natural sea kelp sold as a major bio-catalyst for
bioremediation. Like the Jeneil surfactant, sea kept extract enhances
gram-negatives, while it delays Bacillus germination by 48 hours, rather
than killing them, like the Jeneil product does. The sea kept is useful
for mixed gram formulas for petroleum remediation when the Bacillus are
included to digest fatty acids and other secondary metabolites, so
delaying them until the primary degraders have produced food for them is
useful for that application, but if petroleum degrading Bacillus are
included, slowing them up is not necessarily a good plan.'
comment by Fred. J. Heyrich/Bio-Surge, Inc., NEOTECH
comment by Dave Russell
The problem with swine manure is the high content of sulfur and ammonia. Especially in an anaerobic environment, the conditions are right for formulation of NH3 and H2S which are both particularly stinky. About the best one can do for an anaerobic reaction is to provide covered tankage with either flaring or thermal destruction or alkaline scrubbers to reduce the odors. It is also possible by using perioxides and perhaps permanganates to oxidixe the NH3 and H2S to eliminate them as odor sources.
Odor treatment - What about biofilter treatment
Monday, April 04, 2005
Roundup Highly Lethal To Amphibians in Natural Setting
The news release was originally issued by the University of Pittsburgh - Main Campus and is distributed by AScribe, The Public Interest Newswire.
"The most shocking insight coming out of this was that Roundup, something designed to kill plants, was extremely lethal to amphibians," said Relyea, who conducted the research at Pitt's Pymatuning Laboratory of Ecology. "We added Roundup, and the next day we looked in the tanks and there were dead tadpoles all over the bottom."
"Previous research had found that the lethal ingredient in Roundup was not the herbicide itself, glyphosate, but rather the surfactant, or detergent, that allows the herbicide to penetrate the waxy surfaces of plants. In Roundup, that surfactant is a chemical called polyethoxylated tallowamine. Other herbicides have less dangerous surfactants: For example, Relyea's study found that 2,4-D had no effect on tadpoles."
I expect not the surfactant itself rather the surfactant interacting with the herbicide is the cause - making it bioavailable
Atrazine Runoff
in the beginning of the 1990's when Atrazine was introduced, it was said this herbicide is safe for use and that questioning this is unjustified.
The short term benefit of use does not outweigh the long term risk
Water and Wastewater.com Help Forum - A question about flocculants, help!
aqueous solution is the form the flocculants are applied, or made up at higher concentration before diluting further. The aqueous solutions are prepared either from powder, granular, emulsion form or oil-free dispersion.
That is oil-free dispersion is also a flocculant product sold. The basic problem with highly efficient cationic flocculants is that these are of very high molecular weight with medium to high charge density which results in high viscosity and related problems for preparation of the aqueous solutions.
The most common approach to circumvent this is the emulsion approach.
As far as I know (I hope I remember well) oil-free dispersion means high salt concentration in concentrated flocculant 'solution'.
In normal aqueous environment the polymer chains are in a more or less extended conformation because charged segments repel each other causing high viscosity. Adding high doses of salt will surpress electrostatic interactions and the polyelectolyte molecules coil.
I just do not know how the 'oil-free dispersions' are manufactured, I suppose powder is mixed into the salt solution under high shear. I guess this process requires special know how of the manufacturers.
I think there is no real benefit of using 'oil-free dispersions' instead of emulsions.


